Your Which element has the least metallic character images are available. Which element has the least metallic character are a topic that is being searched for and liked by netizens today. You can Find and Download the Which element has the least metallic character files here. Find and Download all free photos.
If you’re looking for which element has the least metallic character images information related to the which element has the least metallic character keyword, you have pay a visit to the ideal site. Our website frequently gives you suggestions for refferencing the highest quality video and image content, please kindly search and locate more informative video articles and images that match your interests.
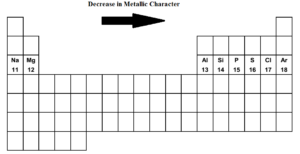
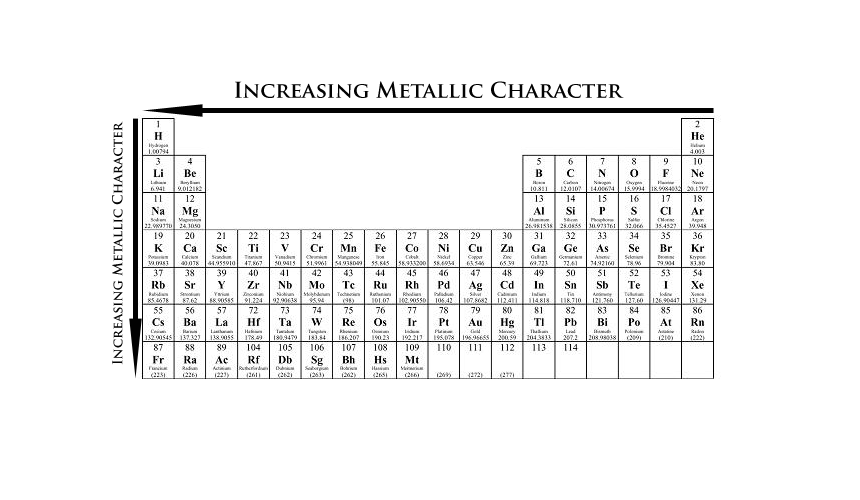
Which Element Has The Least Metallic Character. Carbon phosphorus sulfur bromine and iodine. Now if we analyze the trends in the periodic table metallic character decreases as you move from left to right and from bottom to top of the periodic table. Metallic character increases form right to left across a period on the periodic table and from top to bottom down a group. ASr bAg cSb dXe Fewer valance electrons.
 Metallic And Nonmetallic Character Chemistry For Non Majors From courses.lumenlearning.com
Metallic And Nonmetallic Character Chemistry For Non Majors From courses.lumenlearning.com
Metallic character increases form right to left across a period on the periodic table and from top to bottom down a group. This is because metallic character decreases across a period and increases down a group. What is the least metallic. The electrons of the outermost shell experience less nuclear attraction and so can lose electrons easily thus showing increased metallic character. Fluorine is most non-metallic among F Cl Br and At. Metallic character is mostly dependent on an elements ability to do away its valence electrons.
The element which shows least metallic character is.
What is the least metallic. Non-metals either form negative ions or are unreactive. Which element in period 5 of the Periodic Table is a transition element. Therefore the least metallic elements are the opposite. F C l B r I. The Group 2 alkaline earth metals include Beryllium Magnesium Calcium Barium Strontium and Radium and are soft silver metals that are less metallic in character than the Group 1 Alkali Metals.

Answer 1 of 9. Which is the least metallic alkali metal. Answer 1 of 9. As you see fluorine is the most reactive non metal whereas iodine is the least reactive one. Metallic character is mostly dependent on an elements ability to do away its valence electrons.
 Source: sciencenotes.org
Source: sciencenotes.org
Which element has the LEAST metallic character. The order of non metallic character among halogens is. Carbon phosphorus sulfur bromine and iodine. Which elements have the most metallic character. CarbonThis is because the atomic radius increases and hence it becomes easier to lose electrons.
 Source: pinterest.com
Source: pinterest.com
As you see fluorine is the most reactive non metal whereas iodine is the least reactive one. ASi bAl cNa dMg Ag. Which of these elements has the least attraction for electrons in a chemical bond. Now if we analyze the trends in the periodic table metallic character decreases as you move from left to right and from bottom to top of the periodic table. A metallic compound would have.
 Source: pinterest.com
Source: pinterest.com
The 11 elemental gases are non-metallic with the possible exception of hydrogen which may behave as a. CarbonThis is because the atomic radius increases and hence it becomes easier to lose electrons. Metallic character is mostly dependent on an elements ability to do away its valence electrons. Carbon phosphorus sulfur bromine and iodine. At the same time these metals are still shiny and metallic-looking plus they form cations.
 Source: pinterest.com
Source: pinterest.com
Compared to the atoms of nonmetals in period 3. Which element in period 5 of the Periodic Table is a transition element. Metallic character increases as you move down an element group in the periodic table. A Sr B Ag C Sb D Xe. Of solid and liquid elements at normal temperature and pressure the least metallic are those at upper right of the Periodic Table.
 Source: pinterest.com
Source: pinterest.com
Therefore the least metallic elements are the opposite. Which of these elements has the least attraction for electrons in a chemical bond. A Si B Al C Na D Mg. A silicon B sodium C krypton. Videos you watch may be added to the TVs watch history and influence TV recommendations.
 Source: sciencenotes.org
Source: sciencenotes.org
It was not found as a free element in nature. A Si B Al C Na D Mg. What is the least metallic. The Group 2 alkaline earth metals include Beryllium Magnesium Calcium Barium Strontium and Radium and are soft silver metals that are less metallic in character than the Group 1 Alkali Metals. If playback doesnt begin shortly try restarting your device.
 Source: thefactfactor.com
Source: thefactfactor.com
Non-metals either form negative ions or are unreactive. What is the least metallic. Thus the elements Helium Neon Fluorine. ASr bAg cSb dXe Fewer valance electrons. Of solid and liquid elements at normal temperature and pressure the least metallic are those at upper right of the Periodic Table.

Therefore the least metallic elements are the opposite. The right and uppermost elements on the periodic table. Metallic character increases as you move down an element group in the periodic table. ThalliumHence we can say that thallium has the most metallic character. ASr bAg cSb dXe Fewer valance electrons.
 Source: quora.com
Source: quora.com
Metallic character increases as you move down an element group in the periodic table. The experimentally measured and generally accepted value for the molar volume of Na is about 23 cm3mol. If playback doesnt begin shortly try restarting your device. The electrons of the outermost shell experience less nuclear attraction and so can lose electrons easily thus showing increased metallic character. A Si B Al C Na D Mg.
 Source: pinterest.com
Source: pinterest.com
Which element out of PSCl and F has the least metallic character. Carbon phosphorus sulfur bromine and iodine. Metallic character increases as you move down an element group in the periodic table. Of solid and liquid elements at normal temperature and pressure the least metallic are those at upper right of the Periodic Table. The experimentally measured and generally accepted value for the molar volume of Na is about 23 cm3mol.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Therefore the least metallic elements are the opposite. Which element in period 5 of the Periodic Table is a transition element. Now if we analyze the trends in the periodic table metallic character decreases as you move from left to right and from bottom to top of the periodic table. A Si B Al C Na D Mg. Carbon in some of its allotropic forms has one property in common with metals.
 Source: pinterest.com
Source: pinterest.com
Of solid and liquid elements at normal temperature and pressure the least metallic are those at upper right of the Periodic Table. Carbon phosphorus sulfur bromine and iodine. Carbon in some of its allotropic forms has one property in common with metals. Most metals are malleable and ductile and can be deformed without breaking. So the correct answer is Option B.
 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
As you see fluorine is the most reactive non metal whereas iodine is the least reactive one. The ability of carbon to attract electrons is. Some of the noble metals are brittle rather than malleable. Which is the least metallic alkali metal. As you see fluorine is the most reactive non metal whereas iodine is the least reactive one.
 Source: pinterest.com
Source: pinterest.com
At the same time these metals are still shiny and metallic-looking plus they form cations. It also has a lower electrical conductivity value than other metals. ThalliumHence we can say that thallium has the most metallic character. A metallic compound would have. Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical.
 Source: pinterest.com
Source: pinterest.com
The experimentally measured and generally accepted value for the molar volume of Na is about 23 cm3mol. It was not found as a free element in nature. Which of the following period 3 elements has the least metallic character. Of solid and liquid elements at normal temperature and pressure the least metallic are those at upper right of the Periodic Table. If we look at the periodic table group 17 and group 18 have the least or lowest metallic character.
 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
Metallic character increases form right to left across a period on the periodic table and from top to bottom down a group. Sodium fluoride is an ionic compound because it is composed of a positive and negative ion metal and nonmetal. Which of the following period 3 elements has the least metallic character. The order of non metallic character among halogens is. ASr bAg cSb dXe Fewer valance electrons.
 Source: pinterest.com
Source: pinterest.com
ASi bAl cNa dMg Ag. As an example the metallic character of Beryillium 4 would not be as great as the metallic character of Barium 56. The experimentally measured and generally accepted value for the molar volume of Na is about 23 cm3mol. Which element out of PSCl and F has the least metallic character. Of solid and liquid elements at normal temperature and pressure the least metallic are those at upper right of the Periodic Table.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title which element has the least metallic character by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






