Your Percent ionic character formula images are ready in this website. Percent ionic character formula are a topic that is being searched for and liked by netizens now. You can Download the Percent ionic character formula files here. Find and Download all royalty-free vectors.
If you’re looking for percent ionic character formula images information linked to the percent ionic character formula keyword, you have pay a visit to the ideal blog. Our website frequently provides you with hints for refferencing the maximum quality video and picture content, please kindly search and locate more informative video content and images that fit your interests.
Percent Ionic Character Formula. Therefore to determine the percent ionic character of hydrogen fluoride the measured value of 118 D is divided by 441 D which gives a percent ionic character of 41. Percent ionic character μ observed μ calculated 100. Ea - Eb The subtraction will give a positive value always since modulus modulus sign is used. One method of estimating the percent ionic character is to set it equal to the ratio of the observed dipole moment to the value of eR all multiplied by 100.
 Calculate The Percentage Of Covalent Character Of Hx Having Bond Length 1 62 And Observed Dipole Youtube From youtube.com
Calculate The Percentage Of Covalent Character Of Hx Having Bond Length 1 62 And Observed Dipole Youtube From youtube.com
Percentage of ionic character. Percent ionic character μ observed μ calculated 100. 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A. But the μ of AB is neither zero nor μ ionic. If we remember that 1Å 10 -8 cm then 1D 10 -18 esucm 10 -10 esuÅ. What I shall do now is revert to older units.
The percent ionic character is then just As things stand this is really rather awkward.
Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2. What I shall do now is revert to older units. Percentage ionic character formula In that case the μ of AB would be μ ionic e l 48 10 -18 esu cm. Yet the percent ionic character of Mg-N bonds is 52 48 covalent character. The formula is given below. Using the difference of ionic electronegativity expresseddesignated as XA and XB.
 Source: youtube.com
Source: youtube.com
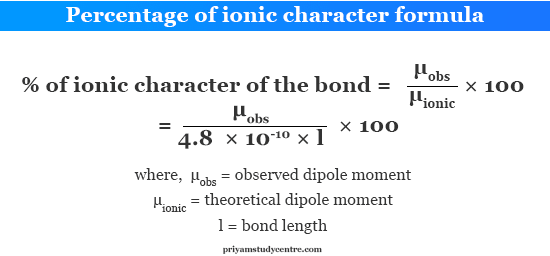
For calculating the dipole moment you need to remember the charge of an electron ie 16 10 19. But from the octet rule HF should have been a purely covalent compound but actually it has some amount of ionic character in it which is due to the electronegativity difference of H and F. Yet the percent ionic character of Mg-N bonds is 52 48 covalent character. The percent ionic character Observed dipole momentCalculated dipole moment assuming 100 ionic bond 100. The formula is written as IC 1-exp -025 XA-XB2100.

Percentage of ionic character. Since the percent ionic character of hydrogen fluoride is less than 50 it is a polar covalent bond. Percentage ionic character formula In that case the μ of AB would be μ ionic e l 48 10 -18 esu cm. Percent ionic character μ observed μ calculated 100. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2.

Ea - Eb The subtraction will give a positive value always since modulus modulus sign is used. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2. μ 100 e l e c t r o n c h a r g e i n t e r a t o m i c s p a c i n g μ 100 16 10 19 C 141 10 10 m μ 100 226 10 29 C m. Materials science and engineering tutorial. Percentage of ionic character 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A.
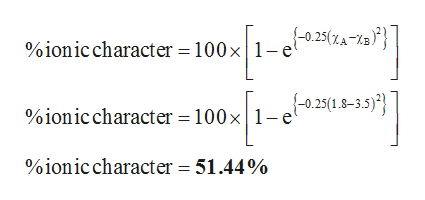 Source: bartleby.com
Source: bartleby.com
One method of estimating the percent ionic character is to set it equal to the ratio of the observed dipole moment to the value of eR all multiplied by 100. Calculate the percent ionic character of HF HCl HBr HI and CsF and compare the results with those in Table 37. A simplistic classification of Mg3N2 as ionic comes from the notion that metals combine with nonmetals to form ionic compounds. A covalent bond with equal sharing of the charge density has 0 ionic character and a perfect ionic bond would of course have 100 ionic character. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables.
 Source: youtube.com
Source: youtube.com
Eb Electronegativity of atom B. Eb Electronegativity of atom B. But Id like to correct the definition of percent ionic character in your question using dipole moment μ not Observed value of ionic character. Percentage of ionic character. Ionic character 1823984 100 4568 When electronegativity difference between two atoms is 21 there is 50 ionic character in the bond.
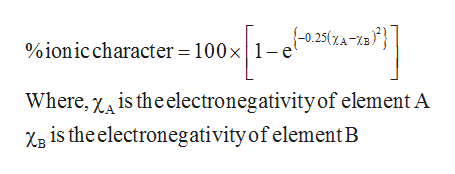 Source: bartleby.com
Source: bartleby.com
If we remember that 1Å 10 -8 cm then 1D 10 -18 esucm 10 -10 esuÅ. Materials science and engineering tutorial. The interatomic distance between k and Cl. A simplistic classification of Mg3N2 as ionic comes from the notion that metals combine with nonmetals to form ionic compounds. The formula is given below.
 Source: toppr.com
Source: toppr.com
Oleum and its percentage labbeling 1 Oleum can be represented by the formula ySO 3 H 2 O where y is the total molar sulphur trioxide content the value of y can be va. One method of estimating the percent ionic character is to set it equal to the ratio of the observed dipole moment to the value of eR all multiplied by 100. Percent ionic character 1 e Δ χ 2 2 100. If we remember that 1Å 10 -8 cm then 1D 10 -18 esucm 10 -10 esuÅ. C Now percentage ionic character exp v a l u e t h e o r e t i c a l v a l u e 100 103 D 61169 D 100 1683 Note.
 Source: youtube.com
Source: youtube.com
Percent ionic character μ observed μ calculated 100. Since the percent ionic character of hydrogen fluoride is less than 50 it is a polar covalent bond. For calculating the dipole moment you need to remember the charge of an electron ie 16 10 19. Why does ionic character increase down group. Ionic character 1823984 100 4568 When electronegativity difference between two atoms is 21 there is 50 ionic character in the bond.
 Source: study.com
Source: study.com
Eb Electronegativity of atom B. Percentage ionic character formula In that case the μ of AB would be μ ionic e l 48 10 -18 esu cm. The formula is given below. As you point out i o n i c c h a r a c t e r μ o b s μ 100 26 10 30 226 10 29. 6 rows Therefore to determine the percent ionic character of hydrogen fluoride measure value of 1.
 Source: clutchprep.com
Source: clutchprep.com
How to determine the percent ionic character. In the alkaline earth metal group down the group as the size increases the ability to lose electrons ie. The percent ionic character Observed dipole momentCalculated dipole moment assuming 100 ionic bond 100. What I shall do now is revert to older units. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables.
 Source: youtube.com
Source: youtube.com
In esu units esu means electrostatic units the fundamental charge is given as e 480320440 1 x 10 -10 esu. Why does ionic character increase down group. Percent ionic character is a measure of compound or molecules ionic and covalent character. But from the octet rule HF should have been a purely covalent compound but actually it has some amount of ionic character in it which is due to the electronegativity difference of H and F. The formula is given below.

Reactivity order of Pyrrole Furan and Thiophene towards Electrophilic substitution. Yet the percent ionic character of Mg-N bonds is 52 48 covalent character. When electronegativity difference is zero identical atoms the bond will be. Using the difference of ionic electronegativity expresseddesignated as XA and XB. Ionic character 1823984 100 4568 When electronegativity difference between two atoms is 21 there is 50 ionic character in the bond.

Why does ionic character increase down group. What I shall do now is revert to older units. The percent ionic character Observed dipole momentCalculated dipole moment assuming 100 ionic bond 100. A covalent bond with equal sharing of the charge density has 0 ionic character and a perfect ionic bond would of course have 100 ionic character. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables.

Reactivity order of Pyrrole Furan and Thiophene towards Electrophilic substitution. Percentage of ionic character 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A. For calculating the dipole moment you need to remember the charge of an electron ie 16 10 19. One method of estimating the percent ionic character is to set it equal to the ratio of the observed dipole moment to the value of eR all multiplied by 100. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables.
 Source: toppr.com
Source: toppr.com
How to determine the percent ionic character. Percentage ionic character of HCl If we consider HCl as a purely ionic compound the charge on hydrogen and chlorine 48 10 -10 esu and bond length 127 10 -8 cm. One method of estimating the percent ionic character is to set it equal to the ratio of the observed dipole moment to the value of eR all multiplied by 100. The percent ionic character Observed dipole momentCalculated dipole moment assuming 100 ionic bond 100. Percent ionic character is a measure of compound or molecules ionic and covalent character.

Eb Electronegativity of atom B. Materials science and engineering tutorial. Why does ionic character increase down group. For calculating the dipole moment you need to remember the charge of an electron ie 16 10 19. C Now percentage ionic character exp v a l u e t h e o r e t i c a l v a l u e 100 103 D 61169 D 100 1683 Note.
 Source: priyamstudycentre.com
Source: priyamstudycentre.com
What I shall do now is revert to older units. Percent ionic character is a measure of compound or molecules ionic and covalent character. How to determine the percent ionic character. The formula is given below. When electronegativity difference is zero identical atoms the bond will be.

C Now percentage ionic character exp v a l u e t h e o r e t i c a l v a l u e 100 103 D 61169 D 100 1683 Note. The percent ionic character Observed dipole momentCalculated dipole moment assuming 100 ionic bond 100. One method of estimating the percent ionic character is to set it equal to the ratio of the observed dipole moment to the value of eR all multiplied by 100. What I shall do now is revert to older units. What is the percent ionic character of Mg3N2.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title percent ionic character formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






